Specificity
- sl10x.in
- July 5, 2024
- No Comments
- 9:02 pm
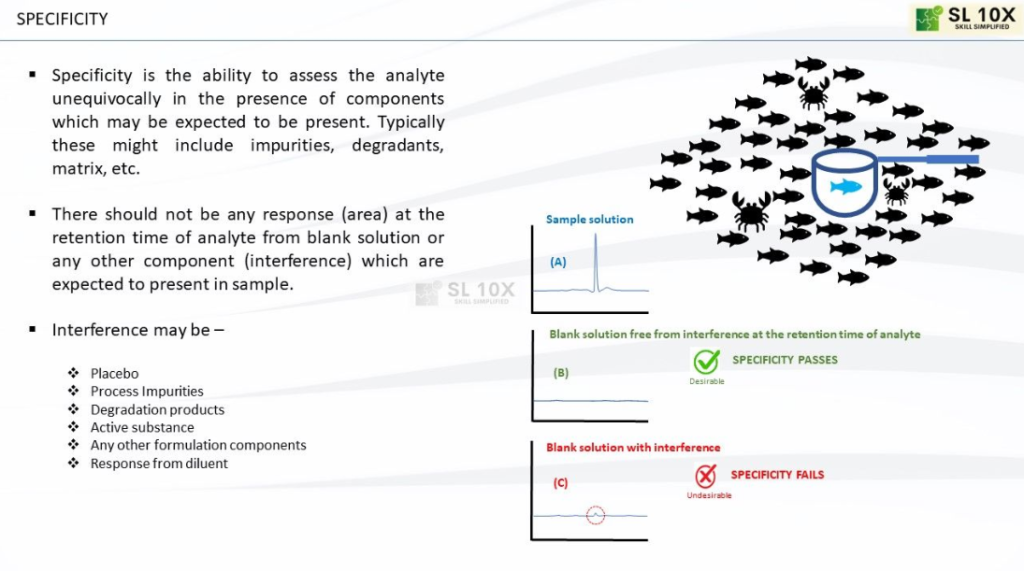
Specificity is a critical parameter in analytical method validation, and it refers to the ability of an analytical method to accurately and selectively measure a particular analyte (compound or substance of interest) in the presence of other components or potential interferents in a sample matrix. In other words, specificity ensures that the method can distinguish and quantify the target analyte without interference from other substances.
Specificity is vital because many real-world samples can be complex, containing various compounds, impurities, or matrix components. Without specificity, the method's results may be inaccurate or biased due to interference from other substances. In pharmaceuticals, for example, ensuring the specificity of an analytical method is crucial to accurately assess the purity of a drug or determine the concentration of an active pharmaceutical ingredient (API) in a formulation.
In some cases, especially in pharmaceuticals, forced degradation studies are conducted. These studies subject the drug product to various stress conditions (e.g., heat, light, acid/base hydrolysis) to generate potential degradation products. Specificity is then demonstrated by showing that the method can accurately measure the analyte in the presence of these degradation products.
ICH guidelines and regulatory agencies often specify acceptance criteria for specificity. These criteria may include peak resolution (for chromatographic methods), absence of interfering peaks, and accurate quantitation of the analyte in the presence of potential interferents. Figure depicts the fundamental aspects of specificity parameter.
sl10x.in
Category Post
Newsletter
Elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
