Van Deemter Equation Demystified
- sl10x.in
- June 23, 2024
- No Comments
- 11:17 am
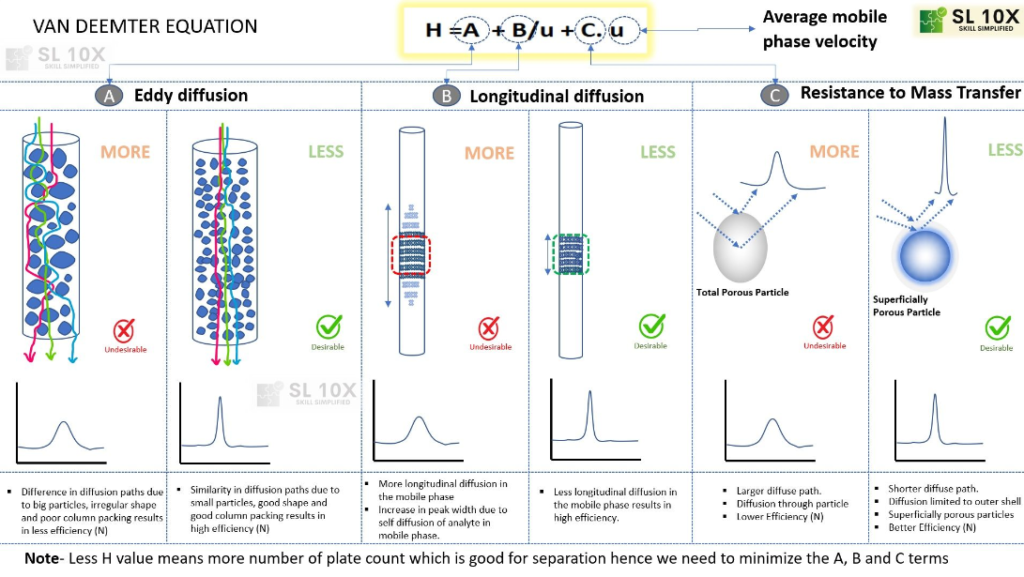
In the realm of High-Performance Liquid Chromatography (HPLC), the injection of a sample initiates as an exceedingly narrow band. However, as the chromatographic process unfolds, this band undergoes broadening. This phenomenon was extensively studied by Van Deemter in the context of Gas Chromatography (GC), leading to the formulation of the Van Deemter equation.
This equation serves to establish a correlation between the Height Equivalent to a Theoretical Plate (HETP) or plate height, and three critical factors influencing band broadening: eddy diffusion, longitudinal diffusion, and resistance to mass transfer.
The Van Deemter equation stands as a foundational tool in chromatography, offering insights into the various mechanisms contributing to the broadening of bands within the chromatographic system. By considering these factors, chromatographers can optimize conditions to minimize band broadening and enhance the efficiency and resolution of separations in HPLC.
Figure provides a visual breakdown illustrating the eddy diffusion, longitudinal diffusion, and mass transfer processes.
sl10x.in
Category Post
Newsletter
Elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
